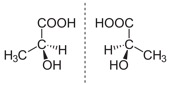
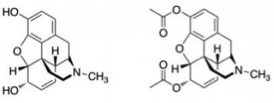
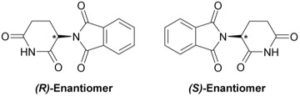
Careers in Science, Technology, Engineering and Mathematics (STEM) have been the fastest growing fields for years now. The employment growth, median wages, and growth opportunities are well above most other fields. “The future of the economy is in STEM,” says James Brown, the executive director of the STEM Education Coalition in Washington, D.C. “That’s where the jobs of tomorrow will be.”
In fact, employment occupations in STEM related fields are expected to grow more than 9 million between 2012 and 2022, and while this is a general projection for all STEM fields, the occupations with the most employment growth are related to technology, math or engineering. Workers in STEM fields earn an average median wage of about $76,000 per year, which is more than double the median wage for all workers.
“STEM offers a cooperative, innovative, and exciting work environment that is unparalleled,” says Aimee Kennedy, vice president for education and STEM learning at Battelle Memorial Institute in Columbus, Ohio.
But how do these “jobs of tomorrow” begin? How do we ensure that more people will choose to go into these fields and contribute great things to the scientific community and society as a whole?
It all begins with education. Preparing for STEM careers can begin as early as high school, and successful STEM workers recommend pursuing challenging courses, such as Advanced Placement (AP) math and science courses, to improve your transcript and prepare for the challenges of STEM work or by taking advantage of free online coding courses.
Most STEM careers require a Bachelor’s degree, and that’s to start. Although a Bachelor’s degree will help you master one field, many career advisors recommended that you use college electives to study other STEM related courses. There are many great careers open to Bachelor’s recipients, despite the common belief that “everyone needs a Ph.D.” Some examples of STEM jobs that require a Bachelor’s are actuaries, civil engineers, and information security analysts.
More advanced jobs, including those in research, usually do require a Master’s or Doctoral degree. A Master’s degree usually requires an additional one to three years after undergraduate study, and most programs require students to write a research paper, known as a thesis paper. Some STEM careers that require a Master’s degree are epidemiologists, hydrologists, and statisticians.
A Doctoral degree usually requires anywhere from three to five additional years after undergraduate study, and students often have to complete a dissertation, which is a lengthy research paper that contributes new knowledge and ideas to their field. Examples of occupations in STEM fields that require Doctoral degrees include animal scientists, computer and information research scientists, and physicists.
Although many jobs require these lengthy studies, there are some STEM related careers that only require an Associate’s degree, and a few require either some college but no degree or a high school diploma or equivalent. An Associate’s degree only requires two years of study, and some examples of occupations in STEM fields that only require an Associate’s include chemical technicians, computer network support specialists, and mechanical drafters.
Work experience in a related field is usually recommended, and sometimes required, for certain STEM careers. Even if your desired occupation does not require experience, it will help to set you apart and help you develop valuable skills before you start. Students need to look for internships and volunteer opportunities while they are still in school. Additionally, getting STEM experience can help you determine exactly what field you want to go into.
Although STEM careers can often be challenging, the rewards far outweigh the disadvantages. Challenges that STEM workers have to face vary depending on the field, but usually involve applying for funding for research, juggling different priorities, navigating government regulations and stressing about deadlines. Despite these downsides, STEM careers often entail problem solving, repeating and refining those problem solving steps, experimentation, building models and using various tools to push ideas from the imagination and turn them into a reality. It also involves writing proposals, so writers and creatives are necessary in the world of STEM to think of new and innovative ways to convey scientific concepts in a way everyone can understand.
STEM workers report being respected and fulfilled. Working in a STEM field will usually mean that you will work on something interesting and meaningful. Most STEM workers find their jobs intellectually stimulating, and they enjoy collaborating with people who share their enthusiasm for science.
Most STEM fields also include rapid change, so the professional development is very dynamic. “There’s always something more to learn,” says Julie Herrick, a volcanologist at the Smithsonian Institution National Museum of Natural History in Washington, D.C. “Don’t expect an end.”
“You feel that what you’re doing is important and you matter as an employee,” says Frances Tirado, a mathematical statistician at BLS in Washington, DC. “People value your skills, listen to your ideas, and think that what you do is magic.” With all these great rewards, advantages, outlooks, and growth, it’s easy to see why careers in STEM are the fastest-growing career field today.
However, according to the Department of Education, only 16% of all High School Seniors are interested in pursuing a STEM career. In order for our society to thrive off our great scientific discoveries and contributions, we need more scientists and engineers. And in order to do so, we need to get children more interested in science and other related subjects–the earlier the better!
Sources:
https://www.nsf.gov/statistics/seind14/index.cfm/chapter-1/c1h.htm
http://money.cnn.com/2014/09/25/smallbusiness/stem-facts/
https://www.bls.gov/careeroutlook/2014/spring/art01.pdf












 Still not convinced of the harmless, beneficial nature of this wildflower? Well how about the industrial uses of goldenrod, which includes being used for rubber in the tires of Model T Fords? Thomas Edison created a cultivation process that maximized the rubber content in goldenrod in order to produce plants that were 12 feet tall and up to 12% rubber. Henry Ford, who was dedicated to finding regenerative properties and alternative crops for materials for his cars, used goldenrod rubber for the tires in the Model T Ford that he gave to Edison as a gift. Later on, during World War II, extensive research and development was conducted to commercialize goldenrod as a source of rubber.
Still not convinced of the harmless, beneficial nature of this wildflower? Well how about the industrial uses of goldenrod, which includes being used for rubber in the tires of Model T Fords? Thomas Edison created a cultivation process that maximized the rubber content in goldenrod in order to produce plants that were 12 feet tall and up to 12% rubber. Henry Ford, who was dedicated to finding regenerative properties and alternative crops for materials for his cars, used goldenrod rubber for the tires in the Model T Ford that he gave to Edison as a gift. Later on, during World War II, extensive research and development was conducted to commercialize goldenrod as a source of rubber. Animal studies have shown that goldenrod can help reduce inflammation, relieve muscle spasms, fight infections and even lower blood pressure. With all of these beneficial side affects, it’s hard to deny the magical, medicinal powers of goldenrod.
Animal studies have shown that goldenrod can help reduce inflammation, relieve muscle spasms, fight infections and even lower blood pressure. With all of these beneficial side affects, it’s hard to deny the magical, medicinal powers of goldenrod.


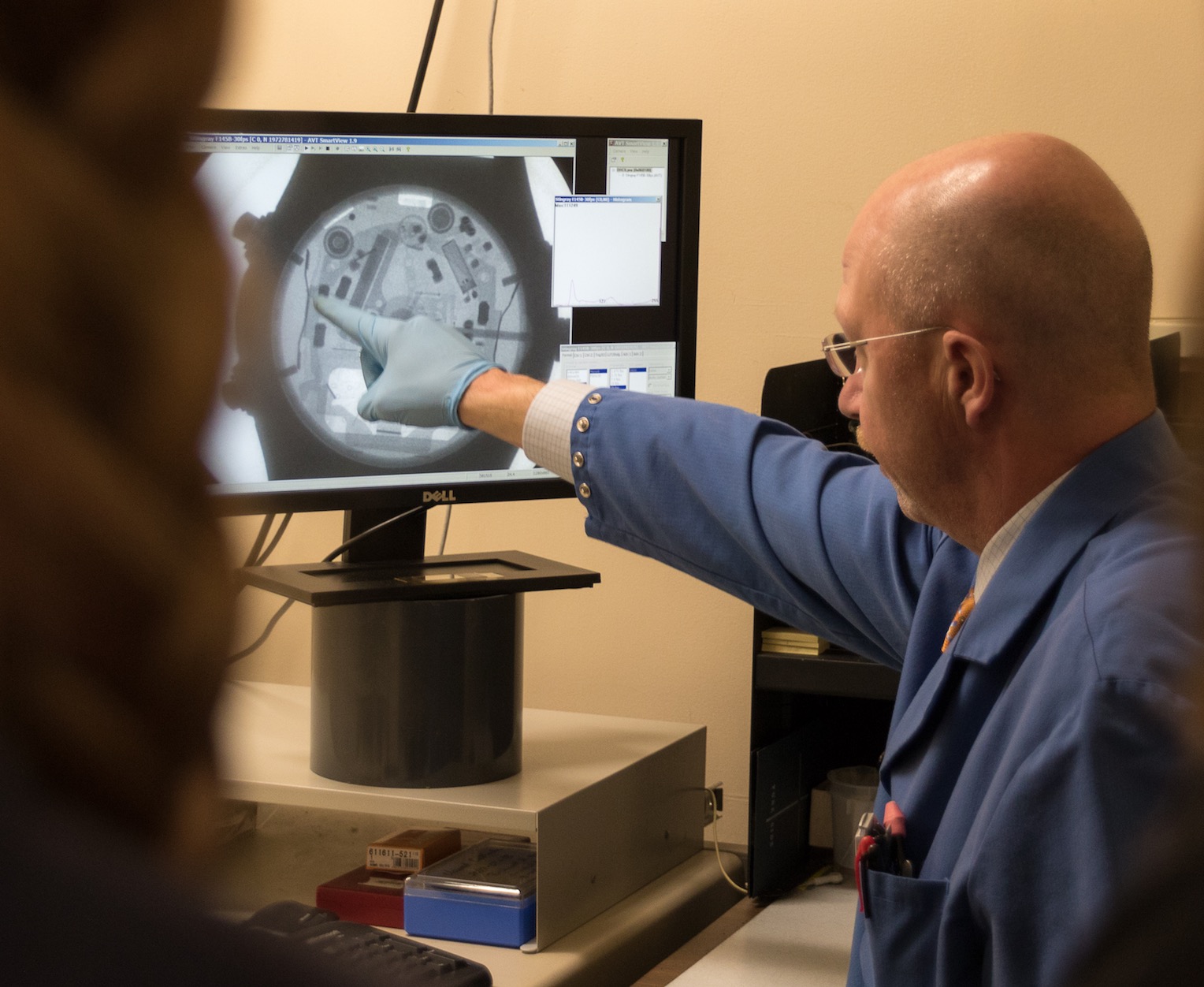
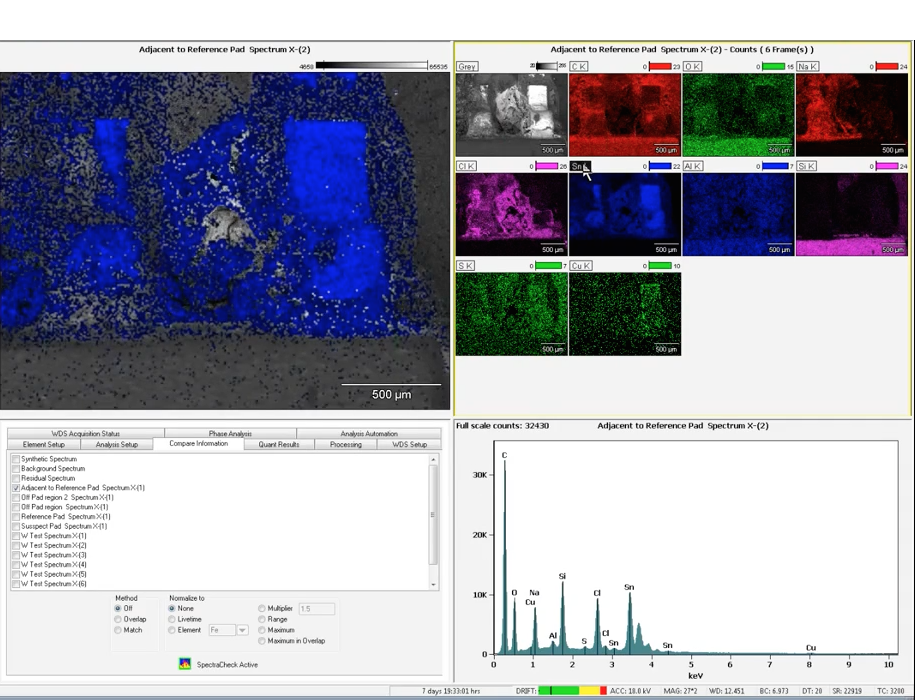
 With a background and a degree in mechanical engineering, working in an analytical lab is almost a seamless transition. Many of the mental gymnastics required of structural and mechanical engineers are close to failure analysis and other tasks at Analytical Answers.
With a background and a degree in mechanical engineering, working in an analytical lab is almost a seamless transition. Many of the mental gymnastics required of structural and mechanical engineers are close to failure analysis and other tasks at Analytical Answers.