Stepping into Jay Powell’s lab at Analytical Answers would overwhelm a person not used to scientific equipment, bright lights and measuring devices. Not that the lab is overly jammed with assorted instruments, but as a Senior Fourier Transform Infrared (FTIR) Spectroscopist Powell’s job requires he master a few disciplines.
While his study is in the field of molecular spectroscopy, the FTIR spectrometer is but a single tool. Mostly because the largest application of molecular spectroscopy is the identification and characterization of organic and polymeric compounds, which often requires application of other instrumentation techniques and devices.
 Powell explains that these include “TGA (Thermogravimetric Analysis), DSC (Differential Scanning Calorimetry), GC (Gas Chromatography), and…classic wet chemical preparation and analysis techniques such as gravimetric and volumetric analysis, pH and ion measurement, Soxhlet extraction, distillation, and others, up to and including organic synthesis.”
Powell explains that these include “TGA (Thermogravimetric Analysis), DSC (Differential Scanning Calorimetry), GC (Gas Chromatography), and…classic wet chemical preparation and analysis techniques such as gravimetric and volumetric analysis, pH and ion measurement, Soxhlet extraction, distillation, and others, up to and including organic synthesis.”
The vast variety of analysis options reflects directly the approach the laboratory takes with all its work. Find the best approach to solve a problem. Powell agrees and his mantra is to select and recommend the best way to analyze a substance or challenge.
Though he got his chemistry Ph.D. in 1984, he hasn’t been so laser focused on scientific discovery to explore other professions – if only to provide himself with perspective on how the world works. It’s a parallel to a lab approach to solving a problem. Learn as much as you can about an item or situation before you attempt to dissect and/or analyze it.
Powell’s experience covers quite a few areas.
“Since starting my career, I’ve been an instructor, researcher, software engineer, applications chemist, instrumentation engineer, product manager, marketing specialist, independent consultant, magazine publisher, accountant, IT specialist, web designer and more,” he said.
Powell remains drawn to chemistry likely because of the influence of his high school chemistry teacher.
“Mr. Massina, had a large influence. He taught all the important things a high school kid wants to know: how to make gunpowder; how to make smoke bombs; how to make contact explosive (nitrogen triiodide),” he said.
Then, in college, Powell discovered his enjoyment of the chemistry work was higher than in other specialties, so he stuck with it. While certainly enamored of scientific discovery, he says not everyone has that love of discovery. When asked what makes science great, Powell shared a philosophical insight.
 “What makes chocolate tasty? What makes a rainbow stunning? What makes a rainbow? What makes the mosquito find you while you’re contemplating the rainbow? How can I make the mosquito go away?” he said. “All of the whats, hows and whys of the physical world is not what makes science great, it is science. Knowing and understanding the details of the physical world allows us to adapt it to our benefit, and to minimize or eliminate those factors which could be to our detriment. What other reason do you need?”
“What makes chocolate tasty? What makes a rainbow stunning? What makes a rainbow? What makes the mosquito find you while you’re contemplating the rainbow? How can I make the mosquito go away?” he said. “All of the whats, hows and whys of the physical world is not what makes science great, it is science. Knowing and understanding the details of the physical world allows us to adapt it to our benefit, and to minimize or eliminate those factors which could be to our detriment. What other reason do you need?”
As Powell continues his scientific exploration on the shiny equipment in the busy lab, you can be sure if there’s a chemical problem to be solved, he’ll find the best approach and then make it happen.
Jay will be speaking on October 18 in our Lunch and Learn Webinar on Polymer Analysis of Biomaterials, Composites,
Encapsulants and Adhesives. This webinar will focus on Infrared Spectroscopy, a technique that measures the light absorbed by the bonds between atoms in a molecule and reveals the molecular structure, which can therefore be used to characterize organic and polymeric samples. You can learn more and register here.




 In ice cream, sugar helps to slow down the freezing process during the churning, which creates the rich and creamy texture we all know and love. In meringues, sugar helps to stabilize the structure. It does this by preventing the egg whites from being over-beaten by slowing down the production of foam, and it protects the foam from collapsing by dissolving in the water bubbles to support them. Sugar can also add a richer, deeper flavor to desserts by caramelizing. When sugar caramelizes, the molecule breaks down into smaller parts and turns a darker color with more complex, richer flavors.
In ice cream, sugar helps to slow down the freezing process during the churning, which creates the rich and creamy texture we all know and love. In meringues, sugar helps to stabilize the structure. It does this by preventing the egg whites from being over-beaten by slowing down the production of foam, and it protects the foam from collapsing by dissolving in the water bubbles to support them. Sugar can also add a richer, deeper flavor to desserts by caramelizing. When sugar caramelizes, the molecule breaks down into smaller parts and turns a darker color with more complex, richer flavors.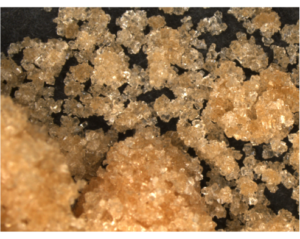 Brown sugar is white sugar that has cane molasses added to it, and comes in either light or dark depending on the amount of molasses added. Brown sugar is better at retaining moisture than white sugar, so it makes baked goods extra moist. “There’s a reason your banana bread is super moist and yummy, it’s that brown sugar and banana!” says Amanda. Unfortunately, brown sugar can harden and clump much easier than white sugar, so it’s a good idea to store it in an airtight container.
Brown sugar is white sugar that has cane molasses added to it, and comes in either light or dark depending on the amount of molasses added. Brown sugar is better at retaining moisture than white sugar, so it makes baked goods extra moist. “There’s a reason your banana bread is super moist and yummy, it’s that brown sugar and banana!” says Amanda. Unfortunately, brown sugar can harden and clump much easier than white sugar, so it’s a good idea to store it in an airtight container.

 So what powers does this mystical compound hold? Specifically, salt can enhance the flavors of other foods by simply increasing the perceived saltiness on our tongues, and it can balance other flavors like sweet and sour and override bitter flavors by suppressing the perception of bitterness. Salt can also denature the rigid structure of proteins, making their flavors tastier and more aromatic, which is why salt and meats go so well together. Salts can bring out aromas by helping release aroma molecules from food into the air, which in turn stimulates our olfactory receptors and helps us smell better. Additionally, as we all know, salt can preserve foods by drawing out the interior moisture of the food and allowing the food to dry out faster, therefore preventing bacteria and mold to grow. This is just a few from the long list of skills of salt.
So what powers does this mystical compound hold? Specifically, salt can enhance the flavors of other foods by simply increasing the perceived saltiness on our tongues, and it can balance other flavors like sweet and sour and override bitter flavors by suppressing the perception of bitterness. Salt can also denature the rigid structure of proteins, making their flavors tastier and more aromatic, which is why salt and meats go so well together. Salts can bring out aromas by helping release aroma molecules from food into the air, which in turn stimulates our olfactory receptors and helps us smell better. Additionally, as we all know, salt can preserve foods by drawing out the interior moisture of the food and allowing the food to dry out faster, therefore preventing bacteria and mold to grow. This is just a few from the long list of skills of salt. Not only does salt have a lot of different skills, but it also has a lot of different types. From table salt to sea salt to Himalayan salt, each of these has a distinct taste and texture, as well as things such as different sodium and mineral contents.
Not only does salt have a lot of different skills, but it also has a lot of different types. From table salt to sea salt to Himalayan salt, each of these has a distinct taste and texture, as well as things such as different sodium and mineral contents. Himalayan salt, or Himalayan pink salt, is harvested from the Khewra Salt Mine in Pakistan, the second largest salt mine in the world. It gets its pink color from small trace amounts of iron oxide, otherwise known as rust. Himalayan salt typically has a slightly smaller sodium chloride concentration, and contains small amounts of calcium, iron, potassium and magnesium. Other than its pink color, however, Himalayan salt is fairly equal to regular salt in regards to taste.
Himalayan salt, or Himalayan pink salt, is harvested from the Khewra Salt Mine in Pakistan, the second largest salt mine in the world. It gets its pink color from small trace amounts of iron oxide, otherwise known as rust. Himalayan salt typically has a slightly smaller sodium chloride concentration, and contains small amounts of calcium, iron, potassium and magnesium. Other than its pink color, however, Himalayan salt is fairly equal to regular salt in regards to taste.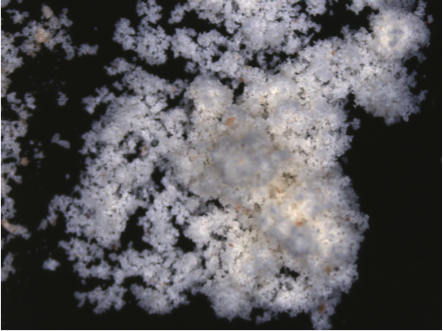
 Flour is an essential ingredient in lots of recipes, mostly for baked goods. But do we really know how flour works? Time after time we mindlessly add flour to our cookies and cakes, overlooking the true importance of it and just waiting for the moment when we get to lick the spoon clean. The truth is, knowing the science behind flour and how it interacts while baking can give us lots of insight about the foods we’re making, the type of flour we should choose for specific recipes, and helpful tips on how to improve these recipes to get the most delicious results.
Flour is an essential ingredient in lots of recipes, mostly for baked goods. But do we really know how flour works? Time after time we mindlessly add flour to our cookies and cakes, overlooking the true importance of it and just waiting for the moment when we get to lick the spoon clean. The truth is, knowing the science behind flour and how it interacts while baking can give us lots of insight about the foods we’re making, the type of flour we should choose for specific recipes, and helpful tips on how to improve these recipes to get the most delicious results. comes to baking. Gluten plays such an important role in baking for many reasons. First of all, it acts as a binding agent for the dough and holds it together. Also, it traps the gases that are released by yeast during fermentation, which prevents bread from being too dense. Furthermore, gluten is ultimately responsible for the shape and texture of baked goods.
comes to baking. Gluten plays such an important role in baking for many reasons. First of all, it acts as a binding agent for the dough and holds it together. Also, it traps the gases that are released by yeast during fermentation, which prevents bread from being too dense. Furthermore, gluten is ultimately responsible for the shape and texture of baked goods. require different levels of gluten content. For example, foods such as breads, pizza dough, pasta and other yeast-raised dough have higher gluten content; the higher amount of gluten makes the breads elastic and stretchy. Other baked goods, such as cakes, cookies and other pastries, require less gluten, which keeps them lighter and fluffy. Therefore, as expected, bread flour has a higher protein content than pastry or cake flour.
require different levels of gluten content. For example, foods such as breads, pizza dough, pasta and other yeast-raised dough have higher gluten content; the higher amount of gluten makes the breads elastic and stretchy. Other baked goods, such as cakes, cookies and other pastries, require less gluten, which keeps them lighter and fluffy. Therefore, as expected, bread flour has a higher protein content than pastry or cake flour. Bread flours, durum semolina and whole-wheat flour have the most protein, about 12-15%. Bread flour is made from hard wheat and forms strong, durable gluten to make the elastic dough necessary for good bread. Whole-wheat flour will provide a more wheat flavor, and is slightly darker than white flour. It is made by milling the whole-wheat berry instead of just parts of it. These flours are therefore ideal for yeast-raised dough and pizzas.
Bread flours, durum semolina and whole-wheat flour have the most protein, about 12-15%. Bread flour is made from hard wheat and forms strong, durable gluten to make the elastic dough necessary for good bread. Whole-wheat flour will provide a more wheat flavor, and is slightly darker than white flour. It is made by milling the whole-wheat berry instead of just parts of it. These flours are therefore ideal for yeast-raised dough and pizzas.