Everyone loves sugar; whether you like just a dash of sugar in your coffee to balance out the bitterness, or a heaping mound of sugar in your desserts to satisfy your insatiable sweet tooth, sugar is an essential ingredient in our everyday lives, especially when it comes to baking. But do we know just how essential it is, and how it all works? Sugar may just be a simple carbohydrate, but simple is the last thing that should be used to describe it. After discovering all the amazing powers that sugar holds, you won’t look at this so-called “simple carbohydrate” the same again!

First of all, let’s take a look at sugar on the molecular level. Sugar is made up of carbon, hydrogen and oxygen, and contains a hydroxyl group, which makes the molecule very polar and therefore very soluble. Sugar also easily bonds with other molecules, and in doing so helps to hold on to the moisture of foods (which also makes it a natural preservative). Common table sugar, and most other sugars, is actually formed by combing two simple sugars and forming a disaccharide, which is great at storing energy.
Now let’s look at how this molecule works and interacts during baking. For example, adding sugar to recipes with flour helps to absorb water and prevent gluten development, which changes the texture of the baked good. The sugar forms strong bonds with water molecules so it helps to keep baked goods soft and moist. Additionally, adding a small amount of sugar will result in a denser texture, such as bread or rolls, and a larger amount of sugar will give a light fluffy texture, such as for cake and other pastries. Sugar also causes cakes and quick breads to rise while baking, because when sugar is mixed with fat, eggs and other liquid ingredients it creates air bubbles, which then expand in the oven causing the batter to rise. Sugar can also provide the crunchy textures to the outside of baked goods. This happens when moisture evaporates from the surface of foods while baking, allowing dissolved sugars to re-crystallize and create crunchy crusts.
 In ice cream, sugar helps to slow down the freezing process during the churning, which creates the rich and creamy texture we all know and love. In meringues, sugar helps to stabilize the structure. It does this by preventing the egg whites from being over-beaten by slowing down the production of foam, and it protects the foam from collapsing by dissolving in the water bubbles to support them. Sugar can also add a richer, deeper flavor to desserts by caramelizing. When sugar caramelizes, the molecule breaks down into smaller parts and turns a darker color with more complex, richer flavors.
In ice cream, sugar helps to slow down the freezing process during the churning, which creates the rich and creamy texture we all know and love. In meringues, sugar helps to stabilize the structure. It does this by preventing the egg whites from being over-beaten by slowing down the production of foam, and it protects the foam from collapsing by dissolving in the water bubbles to support them. Sugar can also add a richer, deeper flavor to desserts by caramelizing. When sugar caramelizes, the molecule breaks down into smaller parts and turns a darker color with more complex, richer flavors.
Clearly, sugar is a very powerful little molecule. But not only does it have this impressive skill set, there are many different kinds that each has its own unique identity. We asked a professional baker and food blogger, Amanda Light (from http://PrimandPropah.com), her opinions on a few different types of sugars. Using her advice, let’s take a look at these main types of sugars and what they bring to the table.
White granulated sugar is what we most commonly associate with the word sugar. It is very refined, so it does not contain any of the natural molasses. It is most commonly used in baking, and because of its extra fine crystals it does not clump together, which makes it easy to sprinkle on top of things or dissolve in liquids. Organic sugar, or brown crystalline, is similar to white sugar but it is less refined so it has a light brown color, and it can be used in the same way as white sugar, according to Amanda.
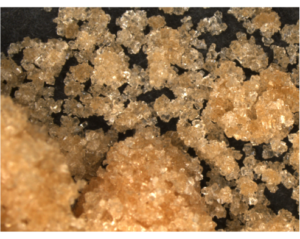 Brown sugar is white sugar that has cane molasses added to it, and comes in either light or dark depending on the amount of molasses added. Brown sugar is better at retaining moisture than white sugar, so it makes baked goods extra moist. “There’s a reason your banana bread is super moist and yummy, it’s that brown sugar and banana!” says Amanda. Unfortunately, brown sugar can harden and clump much easier than white sugar, so it’s a good idea to store it in an airtight container.
Brown sugar is white sugar that has cane molasses added to it, and comes in either light or dark depending on the amount of molasses added. Brown sugar is better at retaining moisture than white sugar, so it makes baked goods extra moist. “There’s a reason your banana bread is super moist and yummy, it’s that brown sugar and banana!” says Amanda. Unfortunately, brown sugar can harden and clump much easier than white sugar, so it’s a good idea to store it in an airtight container.
Liquid sugars, such as honey and agave, are sugars that have been dissolved in water before being used. They hold even more moisture than white or brown sugar, which results in an extra moist texture. Although Amanda says, “I don’t generally bake with honey or agave as my main sugar because I like the consistency of white and brown sugars more,” liquid sugars are great for recipes that first require the sugar to be dissolved.
We hope these facts and tips about sugar will help you in your next baking adventure, and help you to realize the true power of sugar and why it’s so important!
Sources:
http://www.phschool.com/science/biology_place/biocoach/bioprop/polysac.html
http://www.safeeggs.com/blog/how-sweet-it-is-the-science-behind-sugar-in-baking/
http://www.finecooking.com/articles/how-sugar-affects-baking.aspx?pg=0
http://www.mybakingaddiction.com/types-of-sugar/
https://www.sugar.org/all-about-sugar/types-of-sugar/




 With a background and a degree in mechanical engineering, working in an analytical lab is almost a seamless transition. Many of the mental gymnastics required of structural and mechanical engineers are close to failure analysis and other tasks at Analytical Answers.
With a background and a degree in mechanical engineering, working in an analytical lab is almost a seamless transition. Many of the mental gymnastics required of structural and mechanical engineers are close to failure analysis and other tasks at Analytical Answers.

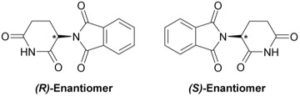

 Powell explains that these include “TGA (Thermogravimetric Analysis), DSC (Differential Scanning Calorimetry), GC (Gas Chromatography), and…classic wet chemical preparation and analysis techniques such as gravimetric and volumetric analysis, pH and ion measurement, Soxhlet extraction, distillation, and others, up to and including organic synthesis.”
Powell explains that these include “TGA (Thermogravimetric Analysis), DSC (Differential Scanning Calorimetry), GC (Gas Chromatography), and…classic wet chemical preparation and analysis techniques such as gravimetric and volumetric analysis, pH and ion measurement, Soxhlet extraction, distillation, and others, up to and including organic synthesis.” “What makes chocolate tasty? What makes a rainbow stunning? What makes a rainbow? What makes the mosquito find you while you’re contemplating the rainbow? How can I make the mosquito go away?” he said. “All of the whats, hows and whys of the physical world is not what makes science great, it is science. Knowing and understanding the details of the physical world allows us to adapt it to our benefit, and to minimize or eliminate those factors which could be to our detriment. What other reason do you need?”
“What makes chocolate tasty? What makes a rainbow stunning? What makes a rainbow? What makes the mosquito find you while you’re contemplating the rainbow? How can I make the mosquito go away?” he said. “All of the whats, hows and whys of the physical world is not what makes science great, it is science. Knowing and understanding the details of the physical world allows us to adapt it to our benefit, and to minimize or eliminate those factors which could be to our detriment. What other reason do you need?”

 In ice cream, sugar helps to slow down the freezing process during the churning, which creates the rich and creamy texture we all know and love. In meringues, sugar helps to stabilize the structure. It does this by preventing the egg whites from being over-beaten by slowing down the production of foam, and it protects the foam from collapsing by dissolving in the water bubbles to support them. Sugar can also add a richer, deeper flavor to desserts by caramelizing. When sugar caramelizes, the molecule breaks down into smaller parts and turns a darker color with more complex, richer flavors.
In ice cream, sugar helps to slow down the freezing process during the churning, which creates the rich and creamy texture we all know and love. In meringues, sugar helps to stabilize the structure. It does this by preventing the egg whites from being over-beaten by slowing down the production of foam, and it protects the foam from collapsing by dissolving in the water bubbles to support them. Sugar can also add a richer, deeper flavor to desserts by caramelizing. When sugar caramelizes, the molecule breaks down into smaller parts and turns a darker color with more complex, richer flavors. Brown sugar is white sugar that has cane molasses added to it, and comes in either light or dark depending on the amount of molasses added. Brown sugar is better at retaining moisture than white sugar, so it makes baked goods extra moist. “There’s a reason your banana bread is super moist and yummy, it’s that brown sugar and banana!” says Amanda. Unfortunately, brown sugar can harden and clump much easier than white sugar, so it’s a good idea to store it in an airtight container.
Brown sugar is white sugar that has cane molasses added to it, and comes in either light or dark depending on the amount of molasses added. Brown sugar is better at retaining moisture than white sugar, so it makes baked goods extra moist. “There’s a reason your banana bread is super moist and yummy, it’s that brown sugar and banana!” says Amanda. Unfortunately, brown sugar can harden and clump much easier than white sugar, so it’s a good idea to store it in an airtight container.

 So what powers does this mystical compound hold? Specifically, salt can enhance the flavors of other foods by simply increasing the perceived saltiness on our tongues, and it can balance other flavors like sweet and sour and override bitter flavors by suppressing the perception of bitterness. Salt can also denature the rigid structure of proteins, making their flavors tastier and more aromatic, which is why salt and meats go so well together. Salts can bring out aromas by helping release aroma molecules from food into the air, which in turn stimulates our olfactory receptors and helps us smell better. Additionally, as we all know, salt can preserve foods by drawing out the interior moisture of the food and allowing the food to dry out faster, therefore preventing bacteria and mold to grow. This is just a few from the long list of skills of salt.
So what powers does this mystical compound hold? Specifically, salt can enhance the flavors of other foods by simply increasing the perceived saltiness on our tongues, and it can balance other flavors like sweet and sour and override bitter flavors by suppressing the perception of bitterness. Salt can also denature the rigid structure of proteins, making their flavors tastier and more aromatic, which is why salt and meats go so well together. Salts can bring out aromas by helping release aroma molecules from food into the air, which in turn stimulates our olfactory receptors and helps us smell better. Additionally, as we all know, salt can preserve foods by drawing out the interior moisture of the food and allowing the food to dry out faster, therefore preventing bacteria and mold to grow. This is just a few from the long list of skills of salt. Not only does salt have a lot of different skills, but it also has a lot of different types. From table salt to sea salt to Himalayan salt, each of these has a distinct taste and texture, as well as things such as different sodium and mineral contents.
Not only does salt have a lot of different skills, but it also has a lot of different types. From table salt to sea salt to Himalayan salt, each of these has a distinct taste and texture, as well as things such as different sodium and mineral contents. Himalayan salt, or Himalayan pink salt, is harvested from the Khewra Salt Mine in Pakistan, the second largest salt mine in the world. It gets its pink color from small trace amounts of iron oxide, otherwise known as rust. Himalayan salt typically has a slightly smaller sodium chloride concentration, and contains small amounts of calcium, iron, potassium and magnesium. Other than its pink color, however, Himalayan salt is fairly equal to regular salt in regards to taste.
Himalayan salt, or Himalayan pink salt, is harvested from the Khewra Salt Mine in Pakistan, the second largest salt mine in the world. It gets its pink color from small trace amounts of iron oxide, otherwise known as rust. Himalayan salt typically has a slightly smaller sodium chloride concentration, and contains small amounts of calcium, iron, potassium and magnesium. Other than its pink color, however, Himalayan salt is fairly equal to regular salt in regards to taste.
 Flour is an essential ingredient in lots of recipes, mostly for baked goods. But do we really know how flour works? Time after time we mindlessly add flour to our cookies and cakes, overlooking the true importance of it and just waiting for the moment when we get to lick the spoon clean. The truth is, knowing the science behind flour and how it interacts while baking can give us lots of insight about the foods we’re making, the type of flour we should choose for specific recipes, and helpful tips on how to improve these recipes to get the most delicious results.
Flour is an essential ingredient in lots of recipes, mostly for baked goods. But do we really know how flour works? Time after time we mindlessly add flour to our cookies and cakes, overlooking the true importance of it and just waiting for the moment when we get to lick the spoon clean. The truth is, knowing the science behind flour and how it interacts while baking can give us lots of insight about the foods we’re making, the type of flour we should choose for specific recipes, and helpful tips on how to improve these recipes to get the most delicious results. comes to baking. Gluten plays such an important role in baking for many reasons. First of all, it acts as a binding agent for the dough and holds it together. Also, it traps the gases that are released by yeast during fermentation, which prevents bread from being too dense. Furthermore, gluten is ultimately responsible for the shape and texture of baked goods.
comes to baking. Gluten plays such an important role in baking for many reasons. First of all, it acts as a binding agent for the dough and holds it together. Also, it traps the gases that are released by yeast during fermentation, which prevents bread from being too dense. Furthermore, gluten is ultimately responsible for the shape and texture of baked goods. require different levels of gluten content. For example, foods such as breads, pizza dough, pasta and other yeast-raised dough have higher gluten content; the higher amount of gluten makes the breads elastic and stretchy. Other baked goods, such as cakes, cookies and other pastries, require less gluten, which keeps them lighter and fluffy. Therefore, as expected, bread flour has a higher protein content than pastry or cake flour.
require different levels of gluten content. For example, foods such as breads, pizza dough, pasta and other yeast-raised dough have higher gluten content; the higher amount of gluten makes the breads elastic and stretchy. Other baked goods, such as cakes, cookies and other pastries, require less gluten, which keeps them lighter and fluffy. Therefore, as expected, bread flour has a higher protein content than pastry or cake flour. Bread flours, durum semolina and whole-wheat flour have the most protein, about 12-15%. Bread flour is made from hard wheat and forms strong, durable gluten to make the elastic dough necessary for good bread. Whole-wheat flour will provide a more wheat flavor, and is slightly darker than white flour. It is made by milling the whole-wheat berry instead of just parts of it. These flours are therefore ideal for yeast-raised dough and pizzas.
Bread flours, durum semolina and whole-wheat flour have the most protein, about 12-15%. Bread flour is made from hard wheat and forms strong, durable gluten to make the elastic dough necessary for good bread. Whole-wheat flour will provide a more wheat flavor, and is slightly darker than white flour. It is made by milling the whole-wheat berry instead of just parts of it. These flours are therefore ideal for yeast-raised dough and pizzas.


